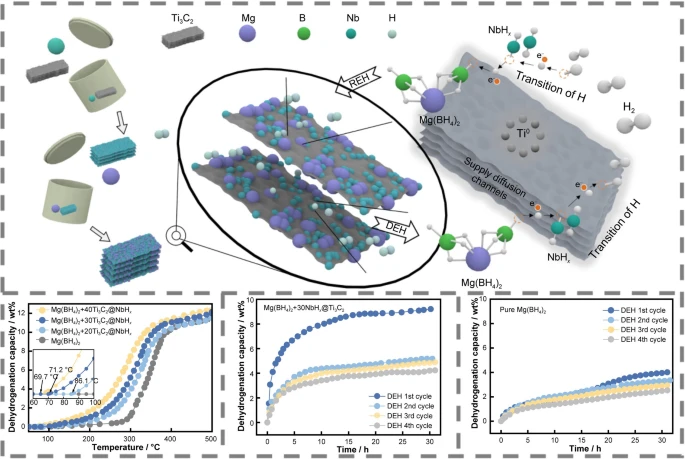
论文简介如下:
为了提高Mg(BH4)2的脱氢动力学和可逆性,采用简易球磨策略实现了Ti3C2负载纳米NbHx颗粒(NbHx@Ti3C2)的构建。NbHx@Ti3C2催化剂的掺杂有效的将Mg(BH4)2的起始脱氢温度由258 °C降至71.2 °C。此外,Mg(BH4)2+30NbHx@Ti3C2复合材料在230 °C的温度下,氢释放量超过9.2 wt%,显示出意想不到的优异脱氢动力学。此外,NbHx@Ti3C2掺杂后Mg(BH4)2的可逆性在四个循环后保持在4.2 wt%以上,与未掺杂Mg(BH4)2相比,可逆性提高了68%。NbHx@Ti3C2催化剂的强催化作用归因于NbHx和Ti3C2在氢气溢出和扩散中的协同作用。
The facile construction of nanoscale NbHx supported by Ti3C2 (NbHx@Ti3C2) was achieved using facile ball milling strategy to improve the hydrogen desorption kinetics and reversibility of magnesium borohydride (Mg(BH4)2). The doping of 30 wt% NbHx@Ti3C2 catalyst reduced the onset dehydrogenation temperature of Mg(BH4)2 to 71.2 °C. Additionally, the Mg(BH4)2 + 30NbHx@Ti3C2 composite achieved remarkable hydrogen release of over 9.2 wt% at a temperature as low as 230 °C, indicating unexpected dehydrogenation kinetics. Moreover, the reversibility of NbHx@Ti3C2 doped Mg(BH4)2 was retained to more than 4.2 wt% after four cycles, which was increased by 68% compared to that of undoped Mg(BH4)2. The strong catalysis of NbHx@Ti3C2 catalyst could be attributed to the synergistic effect of NbHx and Ti3C2 in hydrogen spillover and diffusion. NbHx acted as a “hydrogen pump” for effective hydrogen spillover, while Ti3C2 created numerous diffusion channels for hydrogen dissolution and provided the active catalytic site to facilitate the re/dehydrogenation of Mg(BH4)2.