近期,我院教师郑家广(通讯),硕士生舒裕刚等的研究成果“Hydrogen release of NaBH4 below 60 °C with binary eutectic mixture of xylitol and erythritol additive”在中科院卓越梯队期刊《Chinese Journal of Chemical Engineering》上发表。
论文简介如下:
在21世纪初,硼氢化钠(NaBH4)作为储氢材料在质子交换膜燃料电池和直接硼氢化燃料电池(DBFC)系统等应用中显示出巨大的潜力,因为它具有10.8%(质量)和115 kg/m3的高储氢能力。因此,它被认为是以固态形式储存高密度氢的潜在材料。然而,NaBH4表现出高稳定性,在低于500 °C的温度下很难释放氢气。因此,对NaBH4储氢的主要研究重点之一是降低其脱氢温度。已经探索了几种提高NaBH4脱氢性能的方法,包括催化剂掺杂、纳米限制和反应物不稳定。除了作为储氢介质外,NaBH4还可以作为氢原子(Hδ–)的载体,能够与氢质子(Hδ+)自发反应生成H2。正负氢偶联过程可以利用水和醇作为正氢供体。在过去的十年里,人们进行了大量的研究来探索NaBH4在室温下的水解和醇解。然而,NaBH4-H2O系统用于氢气生产具有一定缺点。首先,NaBH4的水解通常需要昂贵的催化剂。其次,为了实现NaBH4的彻底脱氢,需要大量的水,这会显著降低整个系统的体积氢密度。为了解决这些问题,一种有前途的方法是探索一种能够与NaBH4反应并释放氢气的固体载体,从而避免传统水解方法的缺点。
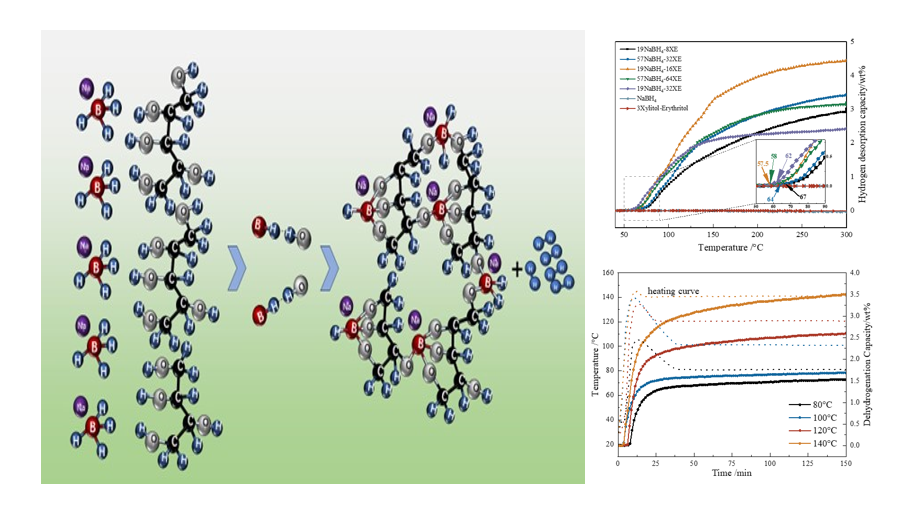
NaBH4 was widely regarded as a low-cost hydrogen storage material due to its high-weight hydrogen capacity of approximately 10.8 wt% and high volumetric hydrogen capacity of around 115 g·L-1. However, it exhibits strong stability and requires temperatures above 500 °C for hydrogen release in practical applications. In this study, two polyhydric alcohols xylitol and erythritol (XE) were prepared as a binary eutectic sugar alcohol through a grinding-melting method. This binary eutectic sugar alcohol was used as a proton-hydrogen carrier to destabilize NaBH4. The 19NaBH4-16XE composite material prepared by ball milling could start releasing hydrogen at 57.5 °C, and the total hydrogen release can reach over 88.8% (4.45 wt%) of the theoretical capacity. When the 19NaBH4-16XE composite was pressed into solid blocks, the volumetric hydrogen capacity of the block-shaped composite could reach 67.2 g·L-1. By controlling the temperature, the hydrogen desorption capacity of the NaBH4-XE composite material was controllable, which has great potential for achieving solid-state hydrogen production from NaBH4.